Equinox Group’s analysis of the Enhertu + Perjeta combination in first-line HER2+ breast cancer predicts a peak-year patient share of 46% in that setting. Our finding is based on initial results from the Destiny-Breast09[1] trial and the expected competitive environment. FDA approval in first-line is likely by the end of 2025[2].
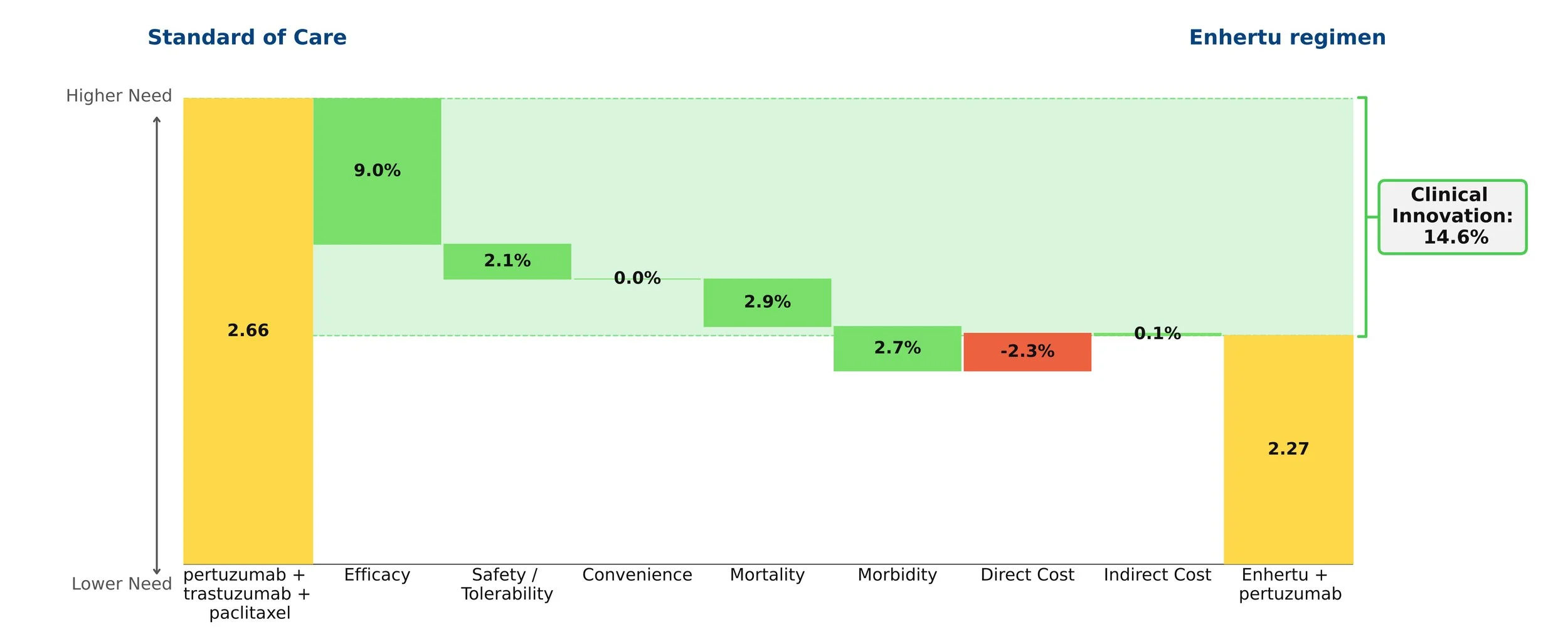
The core of our analysis compares the net clinical improvement of the Enhertu regimen vs. the current standard of care, Perjeta + Herceptin + paclitaxel. Using our rigorous, data-driven methodology, we find that the Enhertu regimen reduces medical need by 14.6%.
History shows that drugs with a 10% or greater reduction in need typically go on to dominate their segment. Enhertu’s improvement in this population is similar to Tagrisso’s advantage in first-line EGFR+ NSCLC.
The graphic quantifies that net clinical improvement, and shows the contribution of each clinical attribute. The biggest driver is efficacy, primarily progression-free survival (40.7 months for the Enhertu regimen vs. 26.9 months for the SOC). Because the median overall survival data is not yet mature, we have made a conservative assumption about that value for Enhertu. Moreover, the Enhertu regimen spares patients paclitaxel, greatly reducing the frequency of neutropenia and offering a cleaner side effect profile. While the Enhertu regimen costs more, that modest disadvantage is overwhelmed by the clinical benefits.