Keytruda (pembrolizumab, Merck) received its 42nd approval from the FDA this Tuesday, February 10th based on the results from the phase 3 KEYNOTE-B96 trial, which looked at the blockbuster PD-1 inhibitor as an add-on to paclitaxel with or without bevacizumab in PD-L1+ platinum-resistant ovarian cancer (PROC). [1] This subset of ovarian cancer patients has developed resistance to standard platinum-based regimens. As a result, they receive non-platinum chemotherapy, such as paclitaxel, pegylated liposomal doxorubicin, or topotecan.
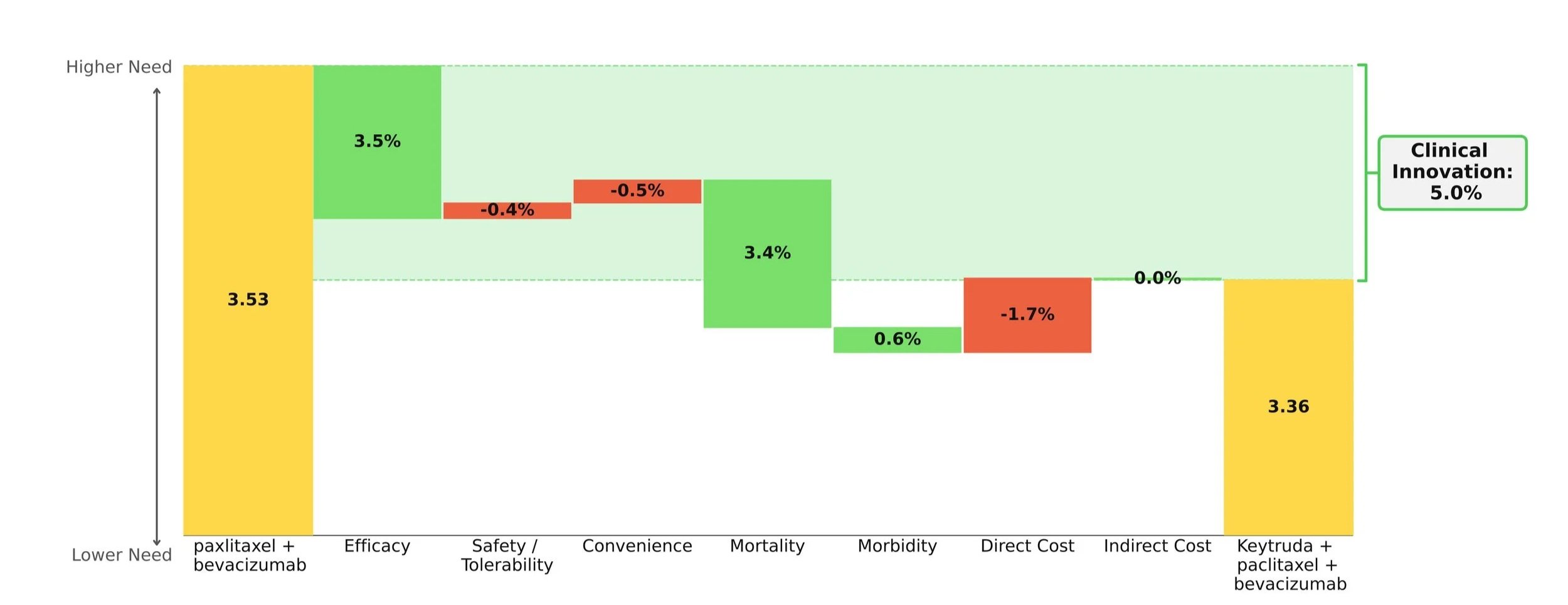
When compared to paclitaxel +/- bevacizumab, the Keytruda regimen showed improvements in survival, progression, and response while maintaining a comparable safety and convenience profile. Importantly, the mortality benefit is what stole the show: an impressive 30% increase in mOS over paclitaxel +/- bevacizumab (19.2 months vs. 14.0 months). [2]
Taking into account the cost impact of adding on Keytruda, the Clinical Innovation is clawed back slightly to a respectable 5.0% overall (Figure 1). Although Keytruda has seen higher levels of innovation elsewhere, such as its many NSCLC indications, a score of 5% typically suggests market differentiation and shows promise for Keytruda's use in this space.
This Clinical Innovation exhibited by Keytruda will increase in the coming years, as Keytruda is scheduled to lose exclusivity in 2028, which will slightly ease the cost burden.
[1] U.S. Food and Drug Administration. FDA approves pembrolizumab with paclitaxel for platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. February 10, 2026. Accessed February 12, 2026.
[2] Cortese T. Pembrolizumab combo significantly improves PFS/OS in recurrent PROC. CancerNetwork. October 18, 2025. Accessed February 12, 2026.