Overview: Avastin (bevacizumab) was the first anti-angiogenesis oncology drug. When it launched in 2004, it was thought that Avastin had virtually limitless potential to be highly effective in a wide range of cancers. More than a decade later, we know that Avastin competed well in some indications, but not others. The level of Clinical Innovation Avastin offers in each of these populations correlates well with the level of commercial success the agent has achieved respectively across indications.
Note: Bubble size reflects size of the patient population
The graphic above compares Avastin in multiple oncology indications for three criteria – level of Clinical Innovation, size of the patient population, and level of unmet medical need. The X axis shows the level of Clinical Innovation Avastin offers in each population; indications right of 0% have positive Clinical Innovation, and those on the left of 0% have negative Clinical Innovation. For instance, Avastin has strong Clinical Innovation (9.2%) in 1st line colorectal cancer. At the other extreme, in 1st line pancreatic cancer, its Clinical Innovation is negative; Avastin never received a label in this population.
The size of the bubble reflects the size of that patient population, and the Y axis reflects the level of medical need (most of these cancer indications have relatively high medical need). Mechanisms with potential in multiple patient segments, such as PD-1 inhibitors, can be assessed in similar ways, given hypotheses for the clinical characteristics of the drug in each of the target populations. This is useful information to inform the prioritization of indications for development. Below we provide more detail comparing Avastin in two of its labeled indications.
Avastin in colorectal cancer:
In 2004, Avastin was approved in 1st line colorectal cancer as an add-on to the standard of care (SOC) FOLFOX6.
There is a strong efficacy advantage in combining Avastin with FOLFOX6 in this population. The improvement in median overall survival (mOS) results in mortality gains; these benefits more than offset the increased cost of adding Avastin to the regimen. The Avastin combination regimen has Clinical Innovation of 9.2% which is good and should therefore become the SOC, which is exactly what occurred.
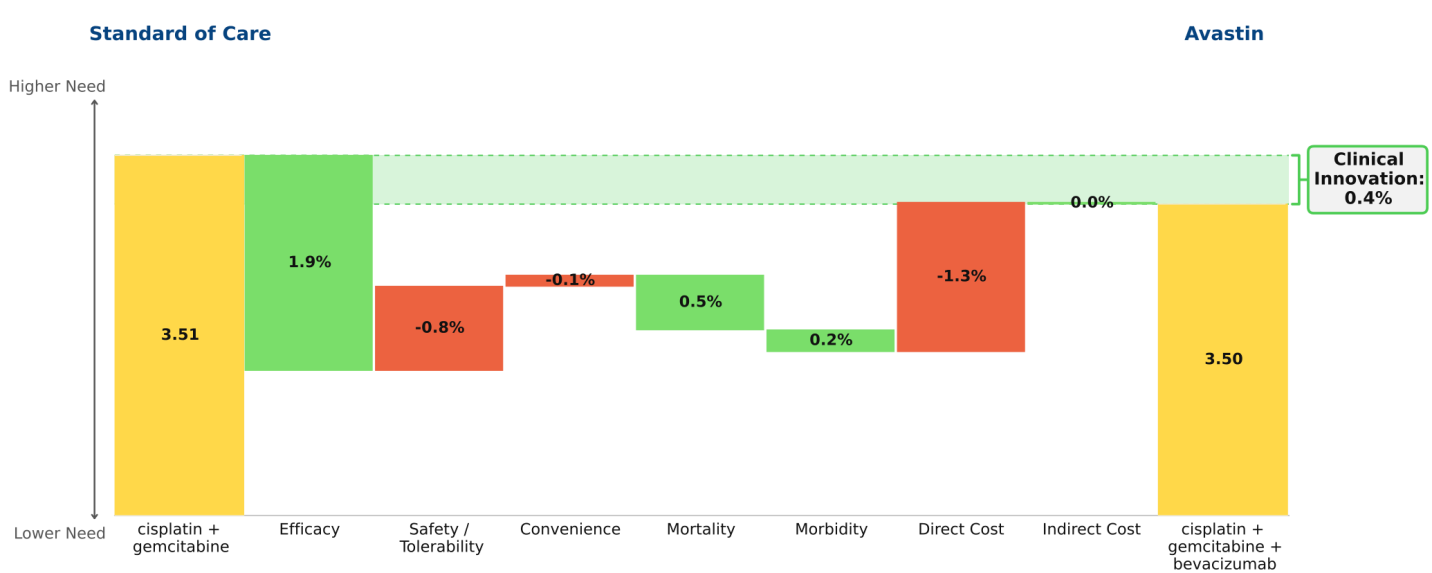
Avastin in non-small cell lung cancer (NSCLC):
Avastin was approved in 1st line NSCLC in 2006, based on a trial in which it was added to a paclitaxel + carboplatin regimen. However, our analytical framework shows that the paclitaxel+ carboplatin regimen is inferior to a cisplatin + gemcitabine regiment, and therefore this latter regimen should be considered the SOC. When we analyze the trial data of gemcitabine + cisplatin with and without Avastin, the Clinical Innovation for the Avastin combination is only 0.4% (“undifferentiated” by our rule of thumb); the efficacy gains are insufficient to offset the increased side effects and drug costs associated with adding Avastin to the gemcitabine combination regimen. This finding explains why Avastin has had low market penetration in this population.