Tools for Early R&D
Disease Target Assessment
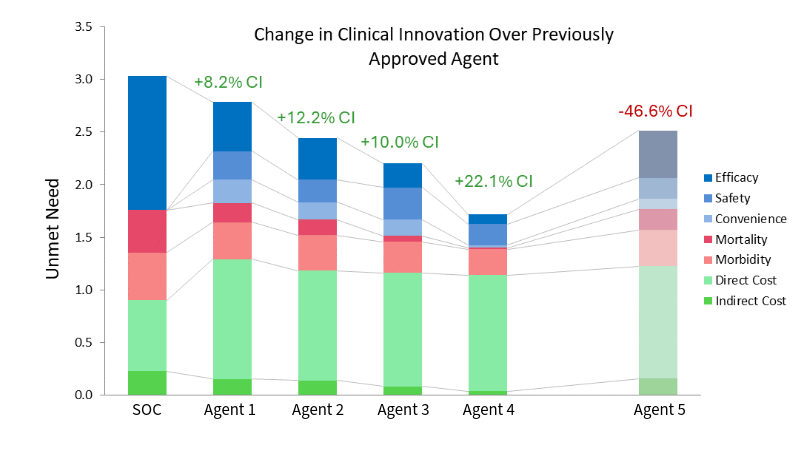
Disease Target Assessment (DTA) is a modeling framework that provides a rigorous and objective measure of the clinical improvement a TPP offers over the standard of care in a specified patient population. We have shown that our measure of clinical improvement (Clinical Innovation) drives patient share, market access, and pricing potential, based on extensive analysis of numerous historical drug launches.
DTA models predict each of those outputs for any modeled TPP and can instantly be updated when new data emerges. Given this dynamic nature, we are able to add on our “Competition Module” functionality when the asset reaches late-stage development, which forecasts the patient share over time of a drug and all its relevant competitors.



Market access
Our market access analytics can be used for both early and late stage assets. We compare the clinical benefit of the drug to its cost, and benchmark those results to a historical data set of launched drugs to identify appropriate analogs. Our clinical benefit vs. cost metric is strongly predictive of the pricing and market access outlook for new agents.





